Olanzapinum
Olanzapinum est substantia antipsychotica et propterea medicamentum ad therapiam schizophreniarum.
 |
Cave: notitiae huius paginae nec praescriptiones nec consilia medica sunt. |
| |

| |
| Natura chemica | |
|---|---|
| Formula chemica | C17H20N4S |
| Massa molaris | 312.439 g/mol |
| PubChem | 4585 |
| DrugBank | DB00334 |
| Natura pharmacologica | |
| Codex ATC | N05AH03 (WHO) |
| Tempus semivitae biologicum | 33 h 51 h (senectute) |
| Metabolismus | iecore (hepaticus): CYP1A2 >> CYP2D6 |
| Excretio | renibus (57%), faecibus (30%) 7% non mutatum |
| Ad usum therapeuticum | |
| Applicatio | per os |
| MedlinePlus | a601213 (Anglice) |
Historia
recensereAb anno 1970 fabricantur diversae substantiae antipsychoticae, atypica quoque vocatae. Inter ea primo Clozapinum fuit, denique ab anno 1971 Olanzapinum, quod usus medicorum ab anno 1996 ad emporium delatum est. Olanzapinum, ut Clozapinum, et receptoria D2 et receptoria serotonini 5-HT1A obsidet, at praeterea minius effectus adversaria observari possunt. Anno 1985 dibenzothiazepino derivata Quetiapinum, simul Clozapino Olanzapinoque simile sed cum grege adiecto, divulgatum est.[1]
Natura Olanzapini
recensereNatura chemica
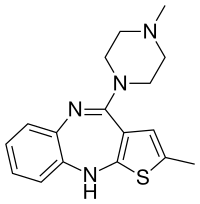
recensereOlanzapinum est piperazinum. Nomen IUPAC suum est 2-methylo-10-(4-methylpiperazin-1-yl)-4H-3-thia-4,9-diazabenzo[f]azulenum. Massa molaris sua 312.439 g/mol.
Natura pharmacologica
recensereOlanzapinum est medicamentum cum virtutibus antipsychoticis. Codex ATC est N05AH03 (WHO).
Pharmacodynamica
recensere| Receptorium | Olanzapini affinitas ligandi, Ki (nM)[2] |
Actio |
|---|---|---|
| dopamini D1 | 35–118 | antagonista |
| D2 | 3.00–106 | |
| D2L | 31–38 | |
| D2S | 21–52 | |
| D3 | 7.8–91 | |
| D4 | 1.6–50 | |
| D4.2 | 17–102 | |
| D4.4 | 21–60 | |
| D5 | 74–90 | |
| 5-HT1A | 2,063–2,720 | antagonista |
| 5-HT1B | 509–660 | nondum determinata |
| 5-HT1D | 540–1,582 | |
| 5-HT1E | 2,010–2,408 | |
| 5-HT1F | 310 | |
| 5-HT2A | 1.32–24.2 | antagonista inversa |
| 5-HT2B | 11.8–12.0 | |
| 5-HT2C | 6.4–29 | |
| 5-HT3 | 202 | antagonista |
| 5-HT5A | 1,212 | agonista |
| 5-HT6 | 6.0–42 | antagonista |
| 5-HT7 | 105–365 | |
| α1A | 109–115 | antagonista |
| α1B | 263 | |
| α2A | 192–470 | |
| α2B | 82–180 | |
| α2C | 29–210 | |
| ß1 | > 10,000 | nondum inquisita |
| ß2 | ||
| H1 | 0.65–4.9 | antagonista inversa |
| H2 | 44 | antagonista |
| H3 | 3,713 | |
| H4 | >10,000 | |
| M1 | 2.5–73 | antagonista |
| M2 | 48–622 | |
| M3 | 13–126 | |
| M4 | 10–350 | |
| M5 | 6.0–82 | |
| σ1 | >5,000 | nondum determinata |
| σ2 | nondum determinata | |
| SERT | ≥3,676 | situm obsidens |
| NET | >10000 | situm obsidens |
| hERG | 6,013 | inhibitor |
Pharmacocinetica
recensereTempus semivitae biologicum .[3] circa 33 h (in senectute 51 h) est. Excretio est per urinas (57%) et biles (30%).
Effectus Olanzapini
recensereCum uso Olanzapini animum advertere ad effectus secundarios et interactiones necesse est.
Effectus secundarii
recensereExempli (!) sunt:
Saepissmime (>10%)
Saepe (1%-10%)
Alia (<1%)
Usus Olanzapini
recensereUsus medicus
recensereOlanzapinum medicamentum pharmaceuticum ad therapiam schizophreniarum est.
Notae
recensere- ↑ Raviña E (2010). The Evolution of drug discovery, Wiley-VCH
- ↑ https://web.archive.org/web/20131108013656/http://pdsp.med.unc.edu/pdsp.php
- ↑ http://goldbook.iupac.org/B00658.html
- ↑ Himmerich H., Minkwitz J., Kirkby K. C. (2015). "Weight Gain and Metabolic Changes During Treatment With Antipsychotics and Antidepressants". Endocrine, metabolic & immune disorders drug targets 15 (4): 252-60
- ↑ Suzuki Y., Ono S., Sugai T., Fukui N., Watanabe J., Tsuneyama N., Sawamura K., Someya T. (2011). "Dose-dependent effects of olanzapine on QT intervals and plasma prolactin levels in Japanese patients with stable schizophrenia". Human psychopharmacology 26 (6): 440-3
- ↑ Waage I. M., Gedde-Dahl A. (2003). "Pulmonary embolism possibly associated with olanzapine treatment". BMJ 327 (7428): 1384