Riluzolum
compositum chemicum
Riluzolum est medicamentum in sclerose laterali amyotrophica[1] tractando.
 |
Cave: notitiae huius paginae nec praescriptiones nec consilia medica sunt. |
| Cognitores | |
|---|---|
| ChemSpider | 4892 |
| PubChem | 5070 |
| DrugBank | DB00740 |
| Natura chemica | |
| |

| |
| Formula summarum | C 8H 5F 3N 2OS |
| Massa molaris | 234.199 g/mol |
| Natura pharmacologica | |
| Codex ATC | N07XX02 (WHO) |
| Tempus semivitae biologicum | 9-15 horae |
| Metabolismus | iecore (hepaticus) |
| Substratum enzymi | CYP1A2 |
| Excretio | renibus: 90% |
| Ad usum therapeuticum | |
| Applicatio | per os |
Natura Riluzoli
recensereNatura chemica
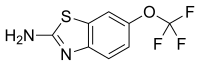
recensereRiluzolum est (IUPAC) 6-(tri-fluor-methoxy)-1,3-benzothiazolum-2-aminum.
Massa molaris est 234.199 g/mol.
Natura pharmacologica
recenserePharmacodynamica
recensereAdhuc machinatio effectuum exacta vacat. Videtur Riluzolum per canales ionticos natrii liberationem glutamati ex terminis tractuum corticoefferentium (imprimis tractus corticospinalis[2]) inhibere.
Pharmacocinetica
recensereExcretio est per imprimis urinas.
Effectus Riluzoli
recensereEffectus secundarii
recensereExempli (!) sunt:
- Nausea
- Fatigatio
- Functio pulmonalis reducta
- Capitis dolor
- Vomitus
- Dolor abdominalis
- Valor aminotransferasis ↑
- Pancreatitis
Nexus interni
Notae
recensere- ↑ Miller R. G., Mitchell J. D., Moore D. H. (Mar 2012). "Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)". The Cochrane database of systematic reviews (3): )CD001447
- ↑ Gorges M., Del Tredici K., Dreyhaupt J., Braak H., Ludolph A. C., Müller H. P., Kassubek J. (Oct 2018). "Corticoefferent pathology distribution in amyotrophic lateral sclerosis: in vivo evidence from a meta-analysis of diffusion tensor imaging data". Scientific reports 8 (1): 15389