Melperonum
|
Cave: notitiae huius paginae nec praescriptiones nec consilia medica sunt. |
| Cognitores | |
|---|---|
| ChemSpider | 14646 |
| PubChem | 15387 |
| DrugBank | DB09224 |
| Natura chemica | |
| |

| |
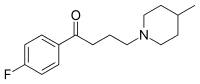
| Formula summarum | C 16H 22FNO |
| Massa molaris | 263.35 g/mol |
| Natura pharmacologica | |
| Codex ATC | N05AD03 (WHO) |
| Tempus semivitae biologicum | 3-4 horae |
| Metabolismus | iecore (hepaticus) |
| Excretio | renibus (70%) |
| Ad usum therapeuticum | |
| Applicatio | per os, i.m. |
Melperonum est substantia sedativum atque antipsychotica levior, ideoque praecipue ad therapiam insomniam praescriptum.
Natura Melperoni recensere
Natura chemica recensere
Melperonum ut benperidolum et haloperidolum et triperidolum est butyrophenonorum (butyrophenonum est 1-phenylbutan-1-onum). Structura chemica melperoni est 4-fluorum-4-(4-methyl-piperidino)-butyrophenonum.
Massa molaris est 263.35 g/mol.
Natura pharmacologica recensere
Melperono effectus sedativus est. Codex ATC est N05AD03.
Pharmacodynamica recensere
Melperonum potissime D3-, deinde alpha2-, alpha1-, D3-, 5-HT2A-, receptoria obsident.
| Receptorium | Affinitas ligandi Ki (nM)[1] |
Annotatio |
|---|---|---|
| serotonini 5-HT1A | 2,200 | |
| serotonini 5-HT1D | 3,400 | |
| serotonini 5-HT2A | 230 | Serotonini receptoriorum affinitas altissima |
| serotonini 5-HT2C | 2,100 | |
| serotonini 5-HT6 | 1,254 | |
| serotonini 5-HT7 | 578 | |
| adrenergici α1 | 180 | |
| adrenergici α2 | 180 | |
| acetylcholini M1 | >10,000 | exigue |
| acetylcholini M2 | 2,400 | |
| acetylcholini M3 | >10,000 | exigue |
| acetylcholini M4 | 4,400 | |
| acetylcholini M5 | >10,000 | exigue |
| dopamini D2 | 194 | Haloperidolum: 1.55 (fortius) |
| dopamini D3 | 8.95 | Dopamini receptoriorum affinitas altissima; Haloperidolum: 0.74 (fortius) |
| dopamini D4 | 555 | |
| histamini H1 | 580 | Haloperidolum: 1,800 (levius) |
Pharmacocinetica recensere
Effectus primi transitus magnus. Tempus semivitae biologicum .[2] 3-4 horae est. Excretio est per urinas et biles.
Effectus Melperoni recensere
Effectus non grati recensere
Cum uso melperoni animum advertere ad effectus secundarios et interactiones necesse est.
Effectus secundarii recensere
Exempli (!) sunt:
- Dystonia
- Akathisia
- Hypersalivatio
- Miosis
- Prolongatio intervalli QT
Interactiones recensere
Nexus interni
Notae recensere
- ↑ PDSP.
- ↑ Goldbook.
- ↑ Gahr, M; Gastl, R; Kölle, MA; Schönfeldt-Lecuona, C; Freudenmann, RW (2012). "Successful treatment of schizophrenia with melperone augmentation in a patient with phenotypic CYP2D6 ultrarapid metabolization: a case report". Journal of Medical Case Reports 6 (1): 49 (Anglice).
- ↑ Köhnke, MD; Lutz, U; Wiatr, G; Schwärzler, F; Weller, B; Schott, K; Buchkremer, G (April 2006). "Cytochrome P450 2D6 dependent metabolization of risperidone is inhibited by melperone". European Journal of Clinical Pharmacology 62 (4): 333–334 (Anglice).
- ↑ Grözinger, M; Dragicevic, A; Hiemke, C; Shams, M; Müller, MJ; Härtter, S (January 2003). "Melperone is an inhibitor of the CYP2D6 catalyzed O-demethylation of venlafaxine". Pharmacopsychiatry 36 (1): 3–6 (Anglice).